Co-Sense for Impurities
Liquid Chromatograph with online Sample Preparation

The FDA draft guidance, "Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches" prescribes permitted limits for genotoxic impurities in drug substances. These impurities demand more sensitive trace-level analysis than normal impurities. Generally, mass spectrometry methods, such as GC/MS or LC/MS, are used for the high-sensitivity analysis of impurities. However, there is increasing demand for the establishment of a high-sensitivity quantitation method using an absorbance or other conventional detector that is easy to operate and can easily apply existing LC analysis conditions.
Features
-
Die Aufnahme von Verunreinigungen ist auf maximal 1,5 µg/Tag beschränkt. bei Einnahme eines Arzneimittels über einen längeren Zeitraum (12 Monate oder länger) Beispiel: Bei einer täglichen Einnahme von 30 mg...
-
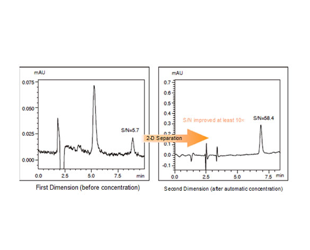
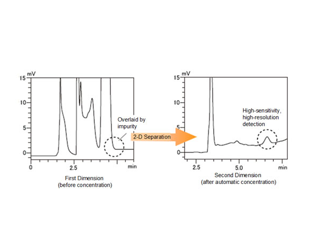
Das Co-Sense System zur Analyse von Verunreinigungn erreicht nur durch... eine etwa 10- bis 20-mal höhere Empfindlichkeit als die 1-D-Trennung.
-
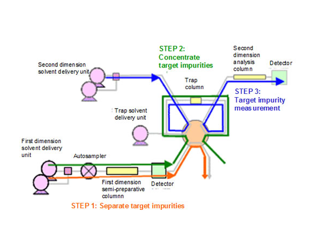
Die Verwendung einer 1-D- und 2-D-Säule mit unterschiedlichen Retentionseigenschaften oder die Verwendung unterschiedlicher Zusammensetzungen der mobilen Phase ermöglicht eine zuverlässige Trennung und ...
-
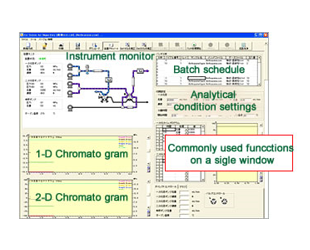
Um die Analysebedingungen festzulegen und die Flusslinien eines 2D-Trennsystems zu reinigen, sind im Allgemeinen erhebliche Kenntnisse und Erfahrungen erforderlich. Jedoch...
Neuigkeiten und Veranstaltungen
-
HPLC Troubleshooting Kurs - Hannover
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden. -
HPLC Troubleshooting Kurs - München
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden. -
HPLC Troubleshooting Kurs - Duisburg II
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden. -
HPLC Troubleshooting Kurs - Berlin
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden. -
HPLC Troubleshooting Kurs - Jena
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden. -
HPLC Troubleshooting Kurs - Darmstadt
In diesem Kurs werden Sie von erfahrenen Kolleg*innen in kleinen Gruppen unterrichtet. Somit können wir auch flexibel auf ihre persönlichen Fragen/Problemstellungen eingehen.
Für diesen Kurs sind 2 Tage vorgesehen (1. Kurstag Online, 2. Kurstag in Präsenz).
Der Theorieteil (Teil 1) wird als interaktive Online-Veranstaltung durchgeführt, der Praxisteil (Teil 2) als Präsenzveranstaltung.
Beide Kurstage folgen nicht unmittelbar aufeinander. Der dazwischen liegende Tag kann somit für die Anreise genutzt werden.